PASylation ® offers several beneficial features, which make this technology attractive for biopharmaceutical drug development:
- The use of PAS sequences is highly versatile ranging from tailored plasma half-life extension over antibody drug conjugates to immunogenicity shielding.
- PAS sequences adopt a stable random conformation under native buffer conditions and at ambient or body temperature and thus generate a large hydrodynamic volume.
- PASylation ® retards renal filtration and prolongs plasma half-life of the biological drug by means of a purely biophysical size effect, without any receptor interactions that may influence pharmacodynamics or cause side effects.
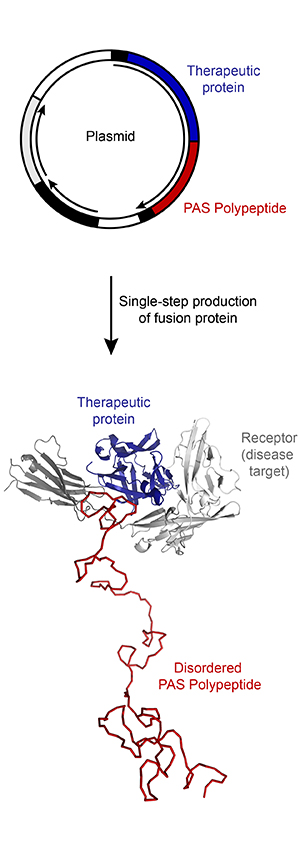
- PAS sequences can be attached via gene fusion to either the N-terminus, the C-terminus or to both termini of a recombinant protein.
- PAS sequences can be adjusted to pharmacological needs by variation of the polypeptide length.
- PAS sequences are resistant against serum proteases while still being degradable by kidney proteases.
- PAS sequences exhibit high solubility without containing charged side chains.
- PAS sequences do not alter the isoelectric point of the biologically active protein.
- PAS sequences are non-toxic, lack T-cell epitopes, and show no signs of immunogenicity in animal experiments.
- PASylation ® avoids the need for additional processing and purification steps due to the simple genetic fusion strategy, which allows biotechnological production and recovery together with the therapeutic protein as one single product.
- PASylation ® has been demonstrated to permit efficient biotechnological production of a series of modified pharmaceutically active proteins in Escherichia coli.